
 |
DNA removal from a purification process of recombinant hepatitis B surface antigen Alejandro Beldarraín Iznaga* Mayda Candelario Frontera Javier Rodríguez Uramis José Blas Tejera González Yodelis Calvo Parra Yoel Madruga González *Corresponding author Financial support: This research was supported by CIGB grants. Keywords: DNA-clearance factor, process characterization, rHBsAg purification process, spiking experiments.
Regulatory agencies states that each unit operation of a cGMP manufacturing process must be controlled to maximize the probability that the finished product meets all quality and design specifications. Biopharmaceutical manufacturers must ensure that their products are free from impurities like nucleic acids, viral particles, in-process intermediate, endotoxins, and host cell proteins (ICH, 1999). DNA considered as cellular contaminant rather than risk factor which required removal to extremely low level and in biopharmaceutical productions, the principal source is host cell DNA. Regulatory authorities challenge manufacturers to reduce the amount of residual DNA in particular in ‘‘well-characterized’’ biopharmaceutical products combining determination of DNA amount in the final product, calculation of clearance factor for each unit operation and demonstration of DNA removal in purification process by clearance experiments with spiked DNA (FDA; European Medicines Evaluation Agency (EMEA) or the World Health Organization (WHO). This study describes the assessment of the DNA removal capacity of an API-rHBsAg manufacturing process combining direct DNA stepwise measurements along downstream purification stream with spiking experiments of two chromatographic separations. Finally, total clearance factor was calculated, demonstrating the high degree of security for an API-rHBsAg purification process to remove DNA burden.
DNA determination By staining with ethidium bromide in agarose gel was performed according to Sambrook et al. 1989. Samples from Acid Precipitation (AP), primary purification material (PP) for manufacturing process, and starting materials and washing for spiking experiments, were analyzed by this method. By dot-blot hybridization was performed by using a radioactivity probe with αdATP32 to quantify residual DNA from P. pastoris host, developed and validated to API-rHBsAg manufacturing process in correspondence to current regulations (Candelario, 1999). Samples from Negative Anion-Exchange chromatography (NAE), Immunoaffinity chromatography (IAF); Desalting by gel filtration chromatography (DS); Positive Anion-exchange chromatography (PAE); Concentration-diafiltration by tangential flow filtration (C-D); Gel filtration chromatography by High Performance Liquid Chromatography (GF) and Active pharmaceutical ingredient (API) for manufacturing process and eluted fractions from spiking experiments, were analyzed by using this method. Chromatographic runs for spiking experiments Chromosomal DNA for spiking experiments, was purified according to procedure described elsewhere (Sambrook et al. 1989). The laboratory scale systems for DNA-clearance studies were performed at 0.1% of manufacturing scale in correspondence to manufacturing procedures (Perez et al. 1994). DNA removal by immunoaffinity chromatography was determined by spiking approx. 5.5 mg rHBsAg with 6 mg purified DNA, equivalent to 2.2 x 107 pg/dose. For ion-exchange chromatography, 18 mg rHBsAg was spiked with 1 mg purified DNA, equivalent to 1.1 x 106 pg/dose. Calculations For further comparison, DNA quantities were expressed in picogram per adult dose of 20 µg rHBsAg according to:
where DNA and rHBsAg represents it respective quantity. The DNA log10 reduction factor (RF) was calculated individually for each operation according to: 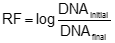 [2] [2]where DNAinitial is the starting DNA quantity in pg/dose and DNAfinal is output DNA quantity after performing purification operation. The total DNA reduction factor was calculated as a sum of the individual log reduction factor. Initially, we investigate DNA removal in manufacturing purification process of API-rHBsAg. Table 1, shown the results obtained during DNA-testing for twenty manufacturing batches, 5 consecutive released in 2000, 2001, 2003 y 2005. DNA removal in small scale spiking experiments for immunoaffinity and positive anion-exchange chromatography To determine the DNA removing capacity of the immunoaffinity and positive anion-exchange chromatography, spiking experiments were carried out at reduced scale under conditions equivalent to those used in the full-scale manufacturing process. Results are shown in Table 2. The efficiency and consistency of a biopharmaceuticals purification process determines drug quality, including which specific types and concentrations of process contaminants may remain. From regulatory point of view, we achieved an essential requirement for API-rHBsAg to conform the commercial vaccine HEBERBIOVAC HB, demonstrating the robustness of manufacturing process to remove DNA in correspondence to current regulations (WHO, 1989; European Pharmacopoeia, 2001a). Results reported in this study are supported for more than 150 million dose released from 1992 up to date without any adverse event reported. CANDELARIO, M. and CALVO, Y. Puesta a punto y validación de un método para cuantificar trazas de DNA. Informe Técnico CIGB, 1999. EUROPEAN
PHARMACOPEIA. Hepatitis B vaccine (rDNA). Council of Europe,
FOOD
AND DRUG ADMINISTRATION, INTERNATIONAL CONFERENCE FOR HARMONIZATION (ICH). Q6B: Specifications: Test procedures and acceptance criteria for biotechnological/biological products. (March, 1999, Geneva, Switzerland). Available from Internet: http://www.fda.gov/Cder/guidance/Q6Bfnl.PDF. PÉREZ,
L.; LÓPEZ, S.; BELDARRAÍN, A.; ARENAL, D. and PENTON, E. Purification
of recombinant Hepatitis B surface antigen (rec-rHBsAg) from P.
Pastoris: A process development study. In: GALINDO, Enrique
and RAMIREZ, Octavio R. eds. Advances in Bioprocess Engineering.
Kluwer Academic Publishers, SAMBROOK, J.; FRITSCH, E. and MANIATIS, T. Molecular cloning: A Laboratory manual. 2nd ed. Cold Spring Harbor Laboratory Press, 1989, 1659 p. ISBN 0-87969-309-6. WORLD HEALTH ORGANIZATION (WHO). Requirement for hepatitis B vaccine made by recombinant DNA technique. Technical report series no. 786, 1989. Available from Internet: http://www.who.int/entity/biologicals/publications/trs/areas/vaccines/hepatitis/WHO_TRS_786_A2.pdf. Note: Electronic Journal of Biotechnology is not responsible if on-line references cited on manuscripts are not available any more after the date of publication. |
|
|