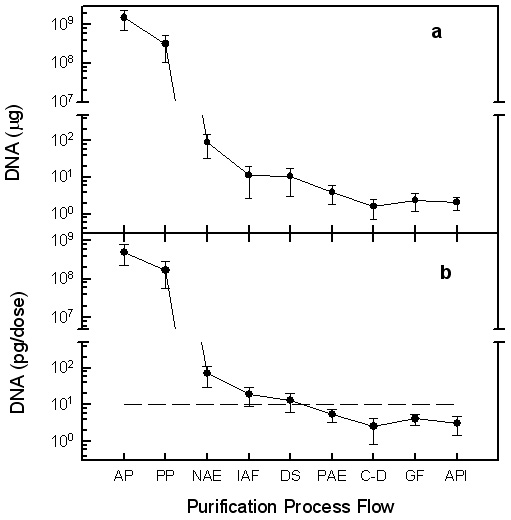
| Figure
1. Removal
of DNA from rHBsAg downstream manufacturing process. The process
operations evaluated were: AP: supernatant of acid precipitation; PP:
material from primary purification; NIE: elution of negative anion-exchange;
IAF: elution of immunoaffinity chromatography; DS: elution of gel filtration
chromatography; PIE: elution of positive anion-exchange; C-D: Concentration-diafiltration
by Tangential Flow Filtration; GF: Gel-Filtration by High Performance
Liquid Chromatography; API: Active Pharmaceutical Ingredient. Dashed
line represents the acceptance limit for API, 10 pg per 20 μg rHBsAg.
(a)
Total DNA in µg.
(b)
Relative DNA in pg/dose HBsAg. |