
 |
Andrés
Illanes Keywords: Biocatalysis,
Biocatalyst, Enzyme inactivation, Enzyme stability, Stabilization
Biocatalysts are inherently
labile; therefore their operational stability is of paramount importance
for any bioprocess. The problem of biocatalyst stability has been tackled
from different perspectives, which are reviewed in the present paper.
Catalysts act by reducing the energy barrier of chemical reactions, therefore producing a dramatic increase in reaction rates, ranging from 106 to 1024 fold. Biocatalysts are strictu sensu the catalysts of cell metabolism, i.e. the enzymes. However, this concept has expanded beyond its physiological meaning, a biocatalyst being, in broader terms, any biological entity capable of catalyzing the conversion of a substrate into a product. Accordingly, biocatalysts can be divided in cellular (whether growing, resting or non-living cells) and non-cellular (enzymes that have been removed from the cell system that produce them). Ribozymes, abzymes and peptide mimics can also be considered as biocatalysts (May, 1992; Benkovic and Ballesteros, 1997). In the context of this article, biocatalysts will refer to enzymes isolated from or conditioned within the cell system that produces them. Living cells, as biocatalysts, are beyond its scope. Biocatalysts have competed and will compete with conventional chemical catalysts. Potential advantages of biocatalysts are their high specificity, high activity under mild environmental conditions and high turnover number; their biodegradable nature and their label as a natural product have become now also very important assets of biocatalysts (Polastro, 1989). Drawbacks are inherent to their complex molecular structure that makes them costly to produce and also intrinsically unstable. Different agents, like temperature and chemicals, promote enzyme inactivation. Inactivation by chemicals can often be avoided rather easily by keeping them out of the reaction medium. Temperature, however, produces opposed effects on enzyme activity and stability and is therefore a key variable in any biocatalytic process (Wasserman, 1984). In fact, both enzymes active at low temperatures and stable at high temperatures, are of great technological potential (Marshall, 1997; Somkuti and Holsinger, 1997). Biocatalyst stability, i.e. the capacity to retain activity through time, is undoubtedly the limiting factor in most bioprocesses, biocatalyst stabilization being then a central issue of biotechnology. In fact, biocatalyst operational stability will determine to a large extent the viability of the process, be it new or faced to compete with an already existing technology. It is reasonable then, that a significant effort in R&D in the field of biocatalysis is devoted, from different perspectives, to enzyme stabilization. Key aspects of biocatalyst stabilization will be reviewed, considering the production of intrinsically stable biocatalysts, strategies for operational stabilization and mathematical modeling of the biocatalyst inactivation during operation.
Strategies for the production of stable biocatalysts Enzyme stability is dictated by its three-dimensional configuration, which is in turn determined by genetic (primary structure) and environmental (interaction with the surroundings) factors. The former aspect will be now addressed. Screening for intrinsically stable biocatalysts is a prominent area of research in biocatalysis. Nature biodiversity is an almost inextinguishable source of biocatalysts. Even though, the systematic exploitation of selected natural niches as sources of enzymes, is rather recent. Research on extremophiles, i.e. organisms able to survive and thrive in extreme environmental conditions, as promising sources for highly stable enzymes is a subject of the present decade (Herbert, 1992; Haard, 1998) and certainly a very active research area at present (Davis, 1998). Those conditions (extreme temperatures, extreme pHs and aggressive chemicals) may be present in the reaction medium, being then fruitful to produce enzymes suited to express and retain their activities in such conditions. Biocatalyst thermostability allows a higher operation temperature, which is clearly advantageous because of a higher reactivity (higher reaction rate, lower diffusional restrictions), higher stability, higher process yield (increased solubility of substrates and products and favorable equilibrium displacement in endothermic reactions), lower viscosity and fewer contamination problems (Mozhaev, 1993). These advantages surmount certain drawbacks arising from more stringent requirements for materials, harder post-reaction inactivation, and restrictions in the case of labile substrates or products. Thermostable biocatalysts are therefore highly attractive. Thermostable enzymes can
be obtained from mesophilic and thermophilic organisms; even psycrophiles
have some thermostable enzymes. Thermophiles represent an obvious source
of thermostable enzymes, being reasonable to assume that such character
will confer their proteins a high thermal stability. This is certainly
so, as can be appreciated in the case of several biotechnologically relevant
enzymes from the hyperthermophilic archaebacteria Pyrococcus furiosus
and Thermotoga sp (Adams et al., 1995; Fischer
et al., 1996, Adams and Kelly, 1998).
In fact, it has been claimed that enzymes from thermophiles are stable at temperatures higher in 20 °C to the optimum growth temperature for such organisms (Daniel, 1996). Interestingly, enzymes from mesophiles exhibit the same pattern. By now, a high number of thermostable enzymes from thermophiles has been reported; most of them belong to eubacterial and archaebacterial kingdoms (Coolbear et al., 1992). However, the technological use of thermophiles still faces several challenges since knowledge on physiology and genetics of such organisms is poor, they are fastidious, grow slowly and are not recognized as safe. Therefore, even though thermal stability can be considered a rare event in mesophilic organisms, thermostable enzymes used by industry are still produced from mesophiles and commercial enzymes from thermophiles are still scarce, as can be appreciated in Table 2 (Kristjánsson, 1989, Coolbear et al.,1992). Opportunities for thermophilic enzymes in industrial processes has been recently highlighted (Caruana, 1997). Table2. Industrial thermostable enzymes, commercial enzymes from thermophiles and termophilic genes cloned in mesophilic hosts
It follows then the convenience of cloning termophilic genes into more suitable mesophilic hosts. Those systems will be highly productive and the enzymes produced will retain its original thermostability. In fact, in a number of cases thermophilic genes have been cloned and expressed in mesophilic hosts, producing enzymes highly active and stable at high temperatures. Some examples are in Table 2 (Kristjánsson, 1989; Adams et al.,1995; Halldórsdóttir, et al., 1998; Rúa et al., 1998). E.coli and other bacterial hosts pose some problems in expressing genes from archibacteria, because of misreading of intervened genes. This is not the case with eubacterial genes, being therefore better candidates for cloning into bacterial hosts (Kristjánsson, 1989). In some cases, remarkable similarities are observed between thermophilic enzymes and their mesophilic counterparts, homology being as high as 85% (Vieille and Zekus, 1996). Thermostability is the result of differences in specific aminoacid sequences and it has been ascribed to a more rigid configuration and to the high number of hydrophobic interactions. By examining primary sequences of termophilic enzymes and mesophilic counterparts the non- conserved regions, as those possibly linked to the thermostable phenotype, can be identified. This opens up the possibility of using protein engineering techniques (Imanaka et al., 1988) to produce point mutations in the mesophilic structural gene, which will result in the corresponding aminoacid substitution in the primary structure of the encoded protein (Daniel, 1996). Good results have been obtained in several cases when replacing aminoacids for those corresponding to the thermophilic protein. Most effective zones for substitution will be the more flexible for being the more labile (Vieille and Zekus, 1996). However, homology between mesophilic enzymes and their thermophilic counterparts are usually between 30 and 50 % and no general strategy for converting mesophilic into thermophilic enzymes have emerged yet, making thermophiles or the genes derived from them the preferred source for thermostable enzymes in the foreseeable future (Adams and Kelly, 1998) Genetic engineering and protein engineering are modern techniques already in use for the commercial production of biocatalysts of improved stability, not only to high temperatures, but also to extremes of pH, oxidizing agents and organic solvents. Cloning and expression in suitable hosts is being used routinely by major enzyme production companies to produce improved biocatalysts; this certainly applies to the cloning of thermostable enzyme genes. Protein engineering is also being used to obtain improved biocatalysts, the case of alkaline protease being a paradigm. Already in the market, thermostable proteases capable to withstand harsh washing conditions (high pH, high concentration of strong oxidants) are products of protein engineering produced by point aminoacid substitutions in the most labile region of the molecule (Anonymous, 1997; Anonymous, 1998). In the production of syrups from cornstarch, thermostability of a -amylase is severely reduced below pH 6, which poses the inconvenience of pH adjustment before and after starch liquefaction. A thermostable a -amylase from Bacillus licheniformis, active at low pH and low Ca++ concentration has been recently patented (Crabb and Mitchinson, 1997). A thermostable glucose isomerase is a major challenge in the production of high-fructose corn syrup. Equilibrium is favored at high temperature, so that at 110 °C 55 % HFCS could be produced at the enzyme reactor stage, without the cumbersome process of sugar fractionation now used (Pedersen, 1993). It was shown that specific substitution of a surface arginine residue for lysine, obtained by site-directed mutagenesis, produced a substantial thermal stabilization in the glucose isomerase from Actinoplanes missouriensis (Quax et al., 1991). Protein engineering is a powerful tool for the design of robust biocatalysts and probably most future biocatalysts will be produced by engineered organisms.
Strategies for the operational stabilization of biocatalysts Biocatalysts are requested to perform in an environment quite different from its natural habitat. Most enzymatic reactions are performed in aqueous media, which favors inactivation. Water acts as a reactant in inactivation reactions and also as a lubricant in conformational changes associated with protein unfolding (Mozhaev, 1993). Therefore, biocatalyst stabilization under operation conditions is a key issue of biocatalysis. Several strategies are at hand to increase operational stability: the use of stabilizing additives, chemical modification of enzyme structure, derivatization, immobilization, crystallization and medium engineering. Use of stabilizing additives is a customary practice in enzyme technology and shelf life of enzyme products very much relies upon such additives. However, its use as operational stabilizers has little significance and poor predictability (Ye et al., 1988), although in the case of enzymes performing in non-conventional media the use of additives has proved to enhance enzyme activity and stability (Triantafyllou et al., 1997). Stabilization by chemical modification of the protein molecule is attractive, but has not received much attention (Inada et al., 1986; O'Fágáin et al., 1988; Besson et al., 1995; Erarslan and Ertan, 1995). Increased stability has been obtained by the introduction of hydrophilic groups in the surface of the enzyme molecule that reduces the contact of hydrophobic regions with water, thereby preventing incorrect refolding after reversible denaturation (Mozhaev, 1993). Derivatization with polymers is being increasingly proposed for the stabilization of soluble enzymes. Modification of proteases with carbohydrate polymers, like polymeric sucrose and dextran, has proven to stabilize them against inactivation induced by temperature and chaotropic agents (Sundaram and Venkatesh, 1998). Horseradish peroxidase has been recently stabilized with several methoxypolyethylene glycols (García and Marty, 1998). Immobilization to solid carriers is perhaps the most used strategy to improve the operational stability of biocatalysts, other benefits being obtained as well, like better control of operation, flexibility of reactor design, and facilitated product recovery without catalyst contamination (Katchalsky-Katzir, 1993). Thermal stability upon immobilization is the result of molecular rigidity and the creation of a protected microenvironment. Among immobilization methods available, multipoint covalent attachment is the most effective in terms of thermal stabilization (Guisán et al., 1993), although thermal stabilization has also been reported for gel-entrapped enzymes (Gianfreda et al., 1985). We have observed dramatic increase in thermal stability by immobilizing different enzymes to glutaraldehyde-activated chitin matrices, where multiple Schiff-base linkages are established between free amino groups in the protein and the aldehyde group in the glutaraldehyde linker (Illanes et al., 1988). Despite its great technological potential, few large-scale processes utilize immobilized enzymes. Severe restrictions may arise because of additional costs, activity losses and diffusional restrictions. In the last few years, improvement in carrier and immobilization techniques are opening new options for process development. In general, immobilized biocatalysts will compete advantageously when the cost of the catalyst is a major component of the processing cost (which is not always the case) and substrates and products are readily soluble and of low molecular weight. Cross-linked enzyme crystals (CLEC) are highly stable novel type biocatalysts. They are produced by stepwise crystallization and molecular cross-linking to preserve the crystalline structure. Uniform size crystals can be obtained in the range from 1 to 100 µm; under 5 µm, diffusional restrictions are insignificant. CLEC are extremely stable, not only with respect to temperature, but also to other inactivating agents like organic solvents. In CLEC, enzyme molecules are compacted almost to the theoretical limit, stabilization being a consequence of intense polar and hydrophobic interactions. Again, molecular rigidity is responsible for thermal stability. Besides, CLEC are highly resistant to proteolysis, proteases being excluded from the tight crystal matrix. Specific activity is significantly higher for CLEC than for immobilized enzymes, although activity can be severely restricted by the low molecular flexibility of the enzyme and substrate size exclusion. Stability of CLEC in organic hydrophobic solvents and water-miscible co-solvents is remarkably high (Noritomi et al., 1998); therefore most applications of CLEC are being developed in connection with enzyme synthesis in organic media. Some examples are the production of chiral compounds, peptides and esters. Several CLEC are now on the market. Some of them are lipases, thermolysin, glucose isomerase and penicillin acylase, the last two of paramount commercial significance (Margolin, 1996). A completely different approach for biocatalyst stabilization is medium engineering, i.e. the manipulation of reaction medium (Gupta, 1992). Since water is involved in enzyme inactivation, partial or almost total substitution of water might be beneficial for biocatalyst stability (Bell et al., 1995). In fact, numerous cases have been reported where remarkable enzyme stability has been obtained in such media (Koskinen and Klibanov, 1996). Until recently, the use of enzymes in non-aqueous media seemed unfeasible because of the very low activities obtained. However, its tremendous technological potential has been a powerful driving force for research and development in that area of biocatalysis, and recent advances are outstanding (Klibanov, 1997). Organic solvents, reputedly incompatible with enzyme activity, have notable exceptions like polyglycols (Khmelnitsky et al., 1988) and glymes and, recently, guidelines for the proper selection of solvents have emerged (Rosell et al., 1998). Even though the main purpose of medium engineering in biocatalysis is associated with the utilization of robust commercial hydrolytic enzymes in organic synthesis (Halling, 1984), thermostability in organic media is an additional bonus of great significance in process economy. In recent years, proteases, lipases, acylases and glycosidases in organic media have been studied in the synthesis of peptides (Feliu et al., 1995; Gill et al., 1996, Sergeeva et al., 1997, Clapés et al., 1997; van Unen et al., 1998), esters (John and Abraham, 1991; Sarney and Vulfson, 1995, Coulon and Ghoul, 1998), oligosaccharides (Bucke, 1996) and glycosides (Stevenson et al., 1993; Scheckermann et al., 1997). Products have considerable pharmacological and industrial relevance and in all those cases thermal stability of the biocatalyst was a key issue. The use of protein engineering to design biocatalysts especially suited to perform in organic solvents is also being studied (Chen et al., 1991). Modeling operational stability of biocatalysts Biocatalyst thermal stability is a fundamental aspect in reactor performance. Despite this, most information on biocatalyst stability, being gathered under non-reactive conditions, is of limited use, leaving aside modulation effects by substrates and products, which certainly play a role during catalysis. Only in few cases, the modulation of enzyme inactivation by reagents and products has been studied and made explicit in reactor modeling (Illanes et al., 1992; Houng et al., 1993, Illanes et al., 1996, Abu-Reesh and Faqir, 1996). Different mechanisms have been proposed to describe enzyme thermal inactivation. The simplest and most used is one-stage first-order kinetics, which proposes the transition of a fully active native enzyme to a fully inactivated species in a single step. Such mechanism leads to a model of exponential decay:
Thermal inactivation is certainly more complex and series and parallel mechanisms have been proposed to describe it (Henley and Sadana, 1986). Models derived from such mechanisms contain a high number of parameters, which are difficult to determine experimentally. However, a two-phase series mechanism usually represents well the phenomenon in terms of a limited number of parameters susceptible to reliable experimental determination. A model based on such mechanism is represented by equation [2]:
where e stands for enzyme activity, e0 is its initial value, t stands for time, k1 and k2 are the transition rate constants in each inactivation stage and A is the specific activity ratio between the intermediate and initial enzyme stage. These models have been traditionally used to evaluate enzyme stability under non-reactive conditions (Ortega et al., 1998), so that parameters obtained do not reflect the behavior in the presence of substrate and products as it occurs in the reactor. It has been postulated that any substance that interacts with the enzyme during catalysis is a potential modulator of enzyme stability (Illanes et al., 1994). Therefore:
where niJ represents the modulation factor of modulator J in inactivation stage i. For instance, in the case of an enzyme subjected to product competitive inhibition, three enzyme species will exist: the free enzyme (E) and the secondary enzyme-substrate (ES) and enzyme -product (EP) complexes, among which the enzyme will be distributed during the course of catalysis. This situation is depicted in the following scheme:
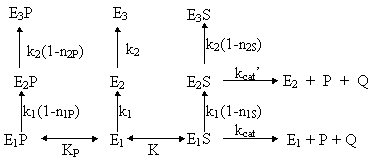 Inactivation parameters can be obtained experimentally by displacing the equilibrium to the enzyme species whose thermal stability is being determined. Equilibrium displacement is achieved by maintaining the enzyme saturated with the modulator during the whole experiment (Illanes et al., 1996). From a material balance on enzyme species and considering two-phase series mechanism of enzyme inactivation (equations [3] and [4]), the following expression is obtained (Illanes et al., 1998a):
where:
Batch reactor performance will be described by solving the system of differential equations [5] and [6], the latter representing the material balance of the reactor: Kinetic and inactivation parameters are temperature dependent, so that reactor operation temperature can be optimized provided that explicit functions of temperature are available for each of them. Rate constants can be conveniently expressed as Arrhenius-type functions:
Affinity parameters, if considered as equilibrium constants, can be expressed as the corresponding thermodynamic correlations: These expressions can be introduced to make equations [5] and [6] explicit in temperature. This will allow the thermal optimization of reactor operation, provided a suitable objective function has been previously defined. Such objective function can be the specific productivity, i.e. the amount of product produced per unit time and enzyme. A cost-based objective function will be the ultimate goal in optimizing the operating temperature. Using experimental data obtained with chitin-immobilized yeast lactase (Illanes et al., 1993), an optimum temperature of 26 °C was obtained when specific productivity was the objective function to maximize (Illanes et al., 1998b). The previous methodology can be extended to any type of reactor operation (continuous fixed-bed and stirred tank) and enzyme kinetic behavior. Models for thermal optimization are of paramount importance to biocatalyst-based processes (Faqir and Abu-Reesh, 1998). This review paper has analyzed the key effects of biocatalyst stability on the optimization of bioprocesses. The present research on intrinsically stable enzymes particularly from extremophiles will have important outcomes for biocatalysis in the near future. Gene cloning of such enzymes on mesophilic hosts and protein engineering of mesophilic enzymes is being exploited already by major enzyme companies to develop stable and robust biocatalysts. Stabilization of biocatalysts by conventional means, like immobilization, and new methodologies, like cross-linked enzyme crystals, is broadening the scope of biocatalysis. Increased stability of enzymes in non-aqueous media is also a relevant technological asset for the development of biocatalysis in organic synthesis. Modeling of operational stability of biocatalysts, considering modulation factors, is required for the proper design of bioreactors. Temperature, as the key variable in such bioprocess, can be conveniently optimized through the use of appropriate models.
Abu-Reesh, I. and Faqir, N. (1996). Simulation of glucose isomerase reactor: optimum operating temperature. Bioprocess Engineering 14:205-210. Adams, M., Perler, F., and Kelly, R. (1995). Extremozymes: expanding the limits of biocatalysis. Bio/Technology 13:662-668. Adams, M. and Kelly, R. (1998). Finding and using hyperthermophilic enzymes. Trends in Biotechnology 16:329-332. Anonymous (1997). Una amplia gama de proteasas, una amplia gama de propiedades. BioTimes (Novo-Nordisk) 2:14-15. Anonymous (1998). Top llega al tope de Japón. BioTimes (Novo-Nordisk) 1:4-5. Bell, G., Halling, P., Moore, B., Partridge,J. And Rees, G. (1995). Biocatalyst behaviour in low-water systems. Trends in Biotechnology 13:468-473. Benkovic, S. and Ballesteros, A. (1997). Biocatalysts- the next generation. Trends in Biotechnology 15:385-386. Besson, C., Favre-Bonvin, G., O'Fagain, C. and Wallach, J. (1995). Chemical derivation of Pseudomonas aeruginosa elastase showing increased stability. Enzyme and Microbial Technology 17:877-881. Bucke, C. (1996). Oligosaccharide synthesis using glycosidases. Journal of Chemical Technology and Biotechnology 67:217-220. Caruana, C. (1997). Enzymes tackle tough processing. Chemical Engineering Progress November, pp 13-20. Chen, K., Robinson, A., Van Dam, M., Martínez, P., Economou, C. and Arnold.H. (1991). Enzymatic engineering for nonaqueous solvents II. Additive effects of mutation on the stabilization and activity of subtilisin E in polar organic media. Biotechnology Progress 7:125-129. Clapés, P., Pera, E. and Torres, J. (1997). Peptide bond formation by the industrial protease, neutrase, in aqueous media. Biotechnology Letters 19:1023-1026. Coolbear, T., Daniel, R. and Morgan, H. (1992). The enzymes from extreme thermophiles: bacterial sources, thermostability and industrial relevance. Advances in Biochemical Engineering/Biotechnology 45:57-98. Coulon, D. and Ghoul, M. (1998). The enzymatic synthesis of non-ionic surfactants: the sugar esters. Agro Food Industry Hi-Tech 9:22-26. Crabb, W. and Mitchinson, C. (1997). Enzymes involved in the processing of starch and sugars. Trends in Biotechnology 15:349-352. Daniel, R. (1996). The upper limits of enzyme stability. Enzyme and Microbial Technology 19:74-79. Davis, M. (1998). Making a living at the extremes. Trends in Biotechnology 16:102-104. Erarslan, A. and Ertan, H. (1995). Thermostabilization of penicillin G acylase obtained from a mutant of E.coli ATCC 11105 by bisimidoesters as homobifunctional cross-linking agents. Enzyme and Microbial Technology 17:629-635. Faqir, N. and Abu-Reesh, I. (1998). Optimum temperature operation mode for glucose isomerase reactor operating at constant glucose conversion. Bioprocess Engineering 19:11-17. Feliu, J., de Mas, C and López-Santín, J. (1995). Studies on papain in the synthesis of Gly-Phe in two-liquid phase media. Enzyme and Microbial Technology 17:882-887. Fischer, L, Bromann, R., Kengen, S., de Vos, W. and Wagner, F. (1996). Catalytic potency of b -glucosidase from the extremophile Pyrococcus furiosus in glucoconjugate synthesis. Bio/Technology 14:88-91. García, D. and Marty, J. (1998). Chemical modification of horseradish peroxidase with several methoxypolyethylene glycols. Applied Biochemistry and Bioengineering 73:173-184. Gill, I., López-Fandiño, R., Jorba, X. and Vulfson, E. (1996). Biologically active peptides and enzymatic approaches to their production. Enzyme and Microbial Technology 18:162-183. Gianfreda, L., Modafferi, M. and Greco Jr, G. (1985). Enzyme stabilization towards thermal, chemical and proteolytic deactivation. Enzyme and Microbial Technology 7:78-82. Guisán, J., Fernández-Lafuente, R., Rodríguez, V., Bastida, A. and Alvaro, G. (1993). Enzyme stabilization by multipoint covalent attachment to activated pre-existing supports. In: Stability and Stabilization of Enzymes (van den Tweel, W., Harder, A., Buitelaar, R., eds). Elsevier, Amsterdam, pp 55-62. Gupta, M. (1992). Enzyme function in organic solvents. European Journal of Biochemistry 203:25-32. Haard, N. (1998). Specialty enzymes from marine microorganisms. Food Technology 52:64-67. Halldórsdóttir,S., Thórólfsdóttir, E., Spilliaert, R., Johansson, M., Thorbjarnardóttir, S., Palsdóttir, A., Hreggvidsson, G., Kristjánsson, J., Holst, O. and Eggertsson, G. (1998). Cloning, sequencing and overexpression of a Rhodothermus marinus gene encoding a thermostable cellulase of glycosilhydrolase family 12. Applied Microbiology and Biotechnology 49:277-284. Halling, P. (1984). Effects of water on equilibria catalysed by hydrolytic enzymes in biphasic reaction systems. Enzyme and Microbial Technology 6:513-516. Henley,J. and Sadana, A. (1986). Deactivation theory. Biotechnology and Bioengineering 28:1277-1285. Herbert, R. (1992). A perspective on the biotechnological potential of extremophiles. Trends in Biotechnology 7:349-353. Houng,J., Yu, H and Chen, K. (1993). Analysis of substrate protection of an immobilized glucose isomerase reactor. Biotechnology and Bioengineering 41:451-158. Illanes, A., Zuñiga, M., Chamy, R. and Marchese, M. (1988). Immobilization of lactase and invertase on crosslinked chitin. In: Bioreactor Immobilized Enzymes and Cells (Moo-Young, M. ed.) Elsevier Applied Science, London, pp 233-249. Illanes, A., Zuñiga, M., Contreras, S. and Guerrero, A. (1992). Reactor design for the enzymatic isomerization of glucose to fructose. Bioprocess Engineering 7:199-204 Illanes, A., Ruiz, A., and Zuñiga, M. (1993). Análisis comparativo de dos lactasas microbianas. Alimentos 18:26-34. Illanes, A., Altamirano, C. and Cartagena, O. (1994). Enzyme reactor performance under thermal inactivation. In: Advances in Bioprocess Engineering (Galindo, E., Ramírez, O. eds.) Kluwer Academic Publishers, Dordrecht, pp 467-472. Illanes, A., Altamirano, C. and Zuñiga, M. (1996). Thermal inactivation of immobilized penicillin acylase in the presence of substrate and products. Biotechnology and Bioengineering 50:609-616. Illanes,
A., Altamirano, C., Aillapán, A., Tomasello, G. and Zuñiga,
M. (1998a). Packed-bed reactor performance with immobilized lactase under
thermal inactivation. Enzyme and Microbial Technology 23:3-9. Imanaka, T., Shibazaki, M. and Takagi, M. (1988). A new way of enhancing the thermostability of proteases. Nature 324:695-687. Inada, Y., Yoshimoto, T., Matsushima, A. and Saito, Y. (1986). Engineering physicochemical and biological properties of proteins by chemical modification. Trends in Biotechnology 4:68-73. John, V. and Abraham, G. (1991). Lipase catalysis and its applications. In: Biocatalysts for Industry (Dordick, J. Ed.) Plenum Press, New York, pp 193-217 Katchalsky-Katzir, E. (1993). Immobilized enzymes - learning from past successes and failures. Trends in Biotechnology 11:471-478. Khmelnitsky, Y., Levashov, A., Klyachko, N. and Martinek, K. (1988). Engineering biocatalytic systems in organic media with low water content. Enzyme and Microbial Technology 10:710-724. Klibanov, A. (1997). Why are enzymes less active in organic solvents than in water? Trends in Biotechnology 15:97-101. Koskinen, A. and Klibanov, A. Enzymatic Reactions in Organic Media. (1996). Blackie Academic & Professional, London, 314 pp. Kristjánsson, J. (1989). Thermophilic organisms as sources of thermostable enzymes. Trends in Biotechnology 10:395-401. Margolin, A. (1996). Novel crystalline catalysts. Trends in Biotechnology 14:223-230. Marshall, C. (1997). Cold-adapted enzymes. Trends in Biotechnology 15:359-364. May, S. (1992). Biocatalysis in the 1990s: a perspective. Enzyme and Microbial Technology 14:80-84. Mozhaev, V. (1993). Mechanism-based strategies for protein thermostabilization. Trends in Biotechnology 11:88-95. Noritomi, H., Koyama, K., Kato, S. and Nagahama, K. (1998). Increased thermostability of cross-linked enzyme crystals of subtilisin in organic solvents. Biotechnology Techniques 12:467-469. O'Fágáin, C., Sheenan, H., O'Kennedy, R. and Kilty, C. (1988). Maintenance of enzyme structure. Possible methods for enhancing stability. Process Biochemistry 23:166-171. Ortega, N., Busto, M. and Pérez-Mateos, M (1998). Stabilisation of b -glucosidase entrapped in alginate and polyacrylamide gels towards thermal and proteolytic deactivation. Journal of Chemical Technology and Biotechnology 73:7-12. Pedersen, S. (1993). Industrial aspects of immobilized glucose isomerase. In: Industrial Application of Immobilized Biocatalysts (Tanaka, A., Tosa, T.,Kobayashi, T., eds). Marcel Dekker, New York, pp 185-208. Polastro, E. (1989). Enzymes in the fine-chemicals industry: dreams and realities. Bio/Technology 7:1238-1241. Quax, W., Mrabet, N., Liiten, R., Schuurhuizen, P., Stanssens, P. and Lasters, I. (1991). Enhancing the thermostability of glucose isomerase by protein engineering. Bio/Technology 9:738-742. Rosell, C., Terreni, M., Fernández-Lafuente, R. and Guisán, J (1998). A criterion for the selection of monophasic solvents for enzymatic synthesis. Enzyme and Microbial Technology 23:64-69. Rúa, M., Atomi, H., Schmidt-Dannert, C. and Schmid, R. (1998). High-level expression of the thermoalkalophilic lipase from Bacillus thermocatenulatus in Escherichia coli. Applied Microbiology and Biotechnology 49:405-410. Sarney, D. and Vulfson, E. (1995). Application of enzymes to the synthesis of surfactants. Trends in Biotechnology 13:164-172. Scheckermann, C., Wagner, F. and Fischer, L. (1997). Galactosylation of antibiotics using the b -galactosidase from Aspergillus oryzae. Enzyme and Microbial Technology 20:629-634. Sergeeva, M., Paradkar, V. and Dordick, J,. (1997). Peptide synthesis using proteases dissolved in organic solvents. Enzyme and Microbial Technology 20:623-628. Somkuti, G. and Holsinger, V. (1997). Microbial technologies in the production of low lactose in dairy foods. Food Science and Technology International 3:163-169. Stevenson, D., Stanley, R. and Furneaux, R. (1993). Optimization of b -D-galactopyranoside synthesis from lactose using commercially available b -galactosidases. Biotechnology and Bioengineering 42:657-666. Sundaram, P. and Venkatesh, R. (1998). Retardation of thermal and urea induced inactivation of a -chymotrypsin by modification with carbohydrate polymers. Protein Engineering 11:699-705. Triantafyllou, A., Wehtje, E., Adlercreutz P. and Mattiasson, B. (1997). How do additives affect enzyme activity and stability in nonaqueous media. Biotechnology and Bioengineering 54:67-76. van Unen, D., Engbersen, J. and Reinhoudt, D. (1998). Large acceleration of a -chymotrypsin-catalyzed dipeptide formation by 18-Crown-6 in organic solvents. Biotechnology and Bioengineering 59:553-556. Vieille, C. and Zeikus, J. (1996). Thermoenzymes: identifying molecular determinants of protein structural and functional stability. Trends in Biotechnology 14:183-190. Wasserman, B. (1984). Thermostable enzyme production. Food Technology 38:78-88. Ye, W., Combes, D. and Monsan, P. (1988). Influence of additives on the thermostability of glucose oxidase. Enzyme and Microbial Technology 10:498-502. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Home | Mail to Editor | Search | Archive |
||