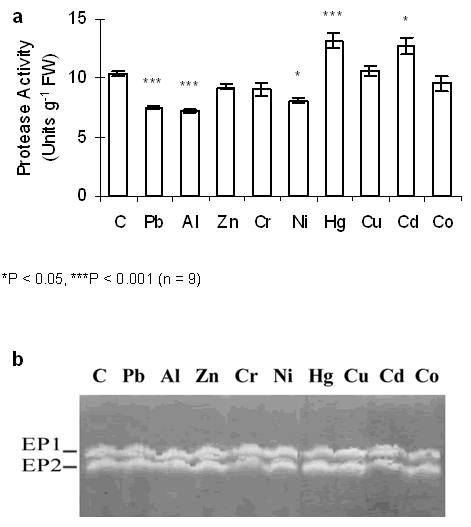
Figure 3. Effect of metals on protease activity.
(a) Azocaseinolytic activity was determined spectrophotometrically by following the digestion of azocasein. One unit of enzyme activity was defined as the difference in absorbance of the azo-dye released at 340 nm after 1 hr of incubation.
(b) Gelatinase profile. Detection of protease activity in SDS-PAGE. Cotyledon extracts (50 µg of total protein) were subjected to SDS-PAGE (10% (w/v) polyacrylamide, contained 0.10% (w/v) gelatin). After electrophoresis, gels were incubated in citrate buffer 100 mM (pH 5.2) at 40ºC overnight. Following incubation, gels were stained with amido black solution. Protease activity was detected on a dark-blue background as colourless bands. Gels were photographed with a Fotodyn, analyzed with GelPro software and expressed arbitrary units based on absolute integrated optical density of each band.